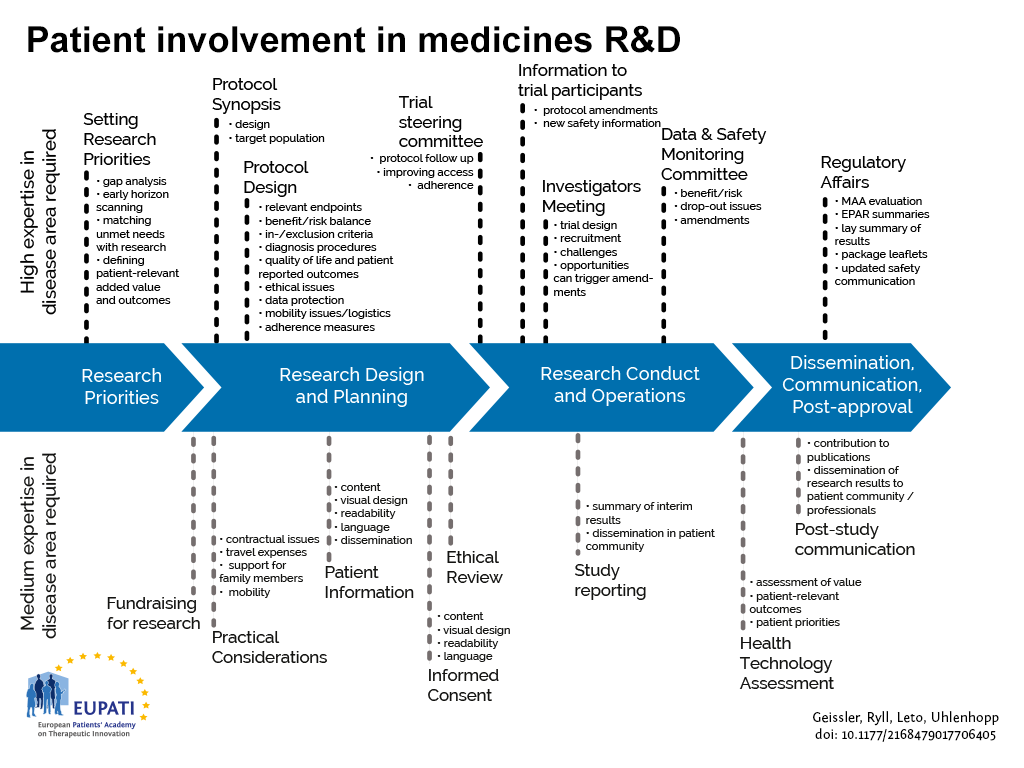
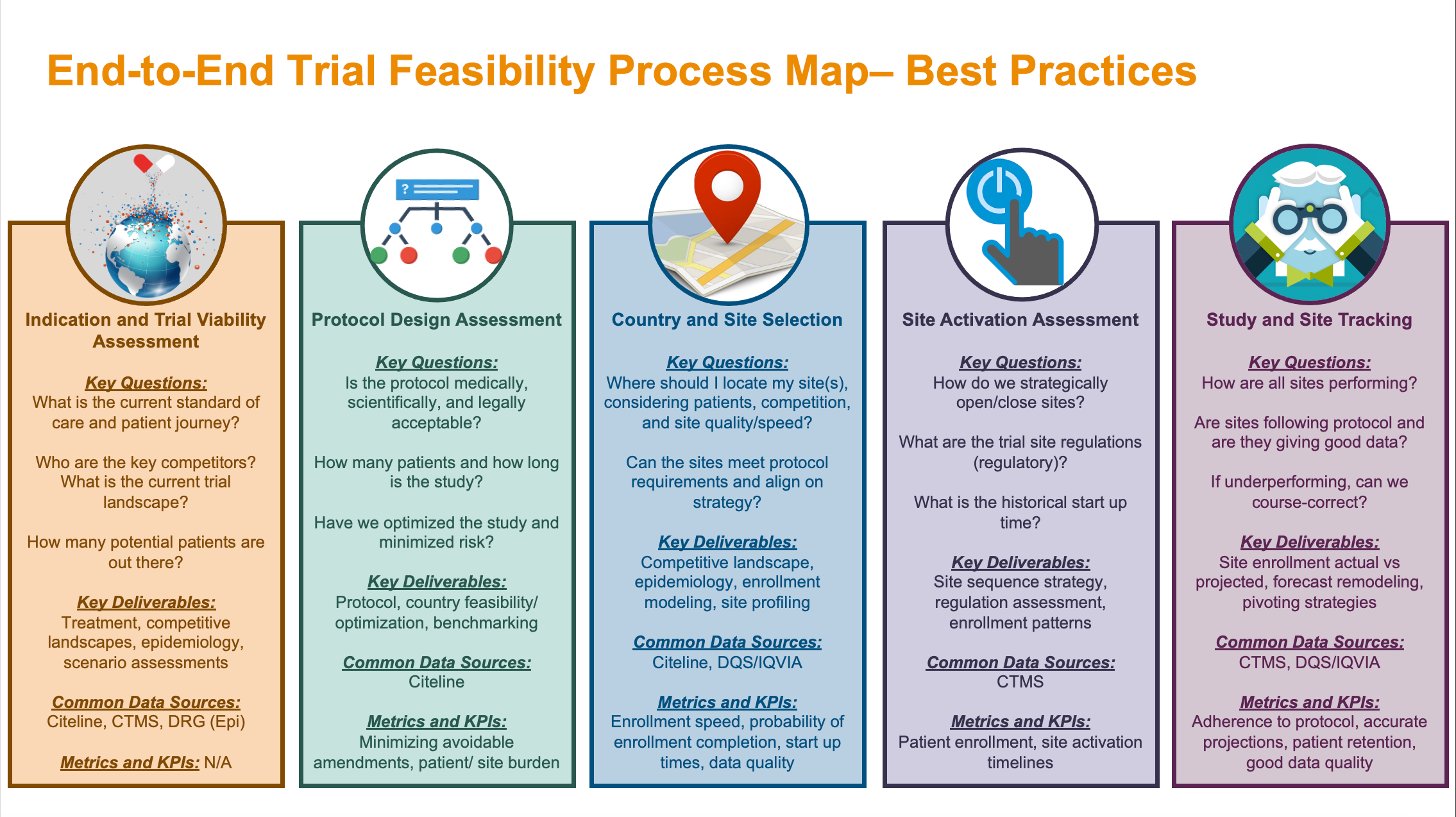
SOPs for GCP-Compliant Clinical Trials: A Customizable Manual for Sponsors of Medical Device Trials : MS Word Template | CenterWatch

PDF) Clinical investigation plan for the use of interactive binocular treatment (I-BiT) for the management of anisometropic, strabismic and mixed amblyopia in children aged 3.5–12 years: a randomised controlled trial