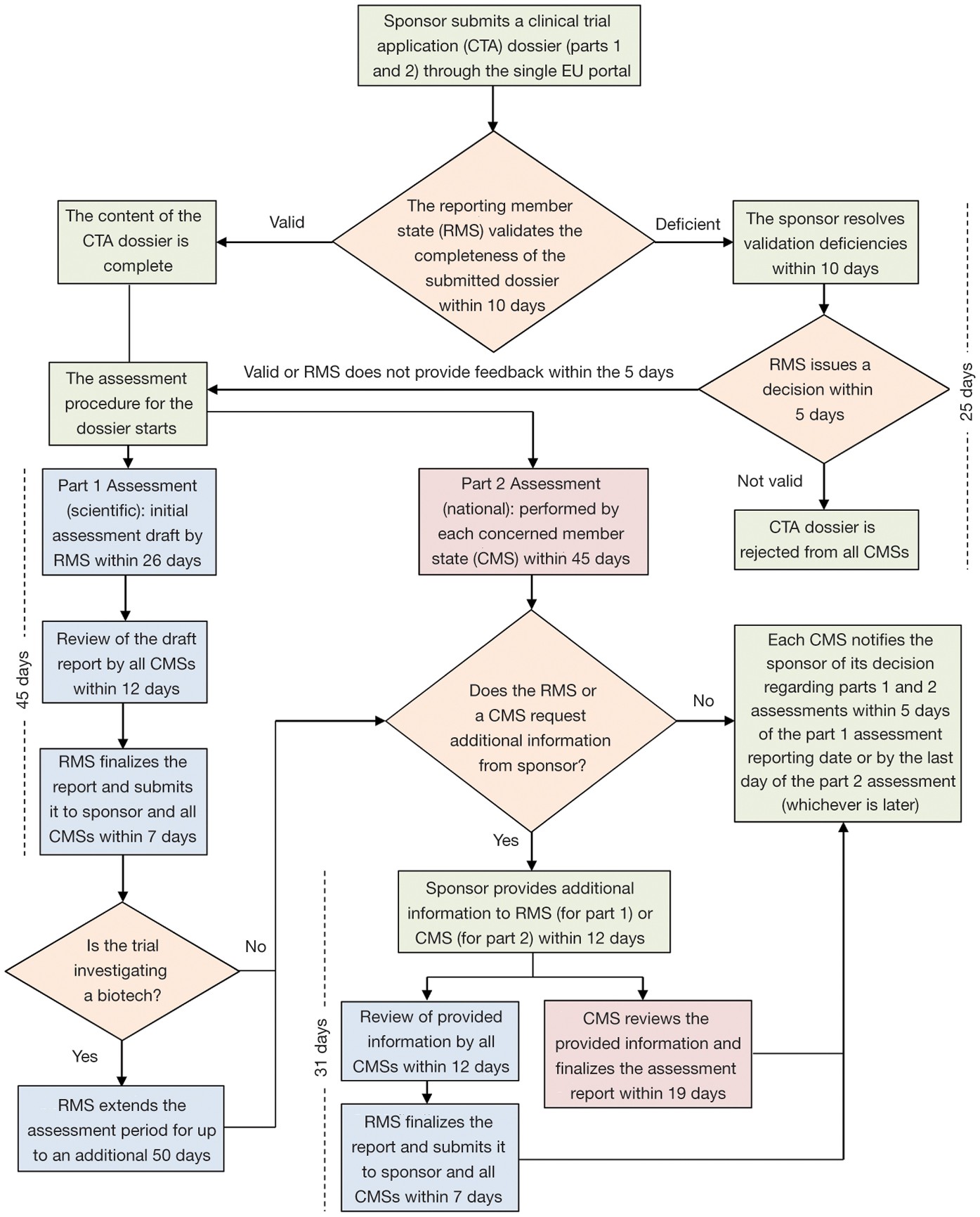
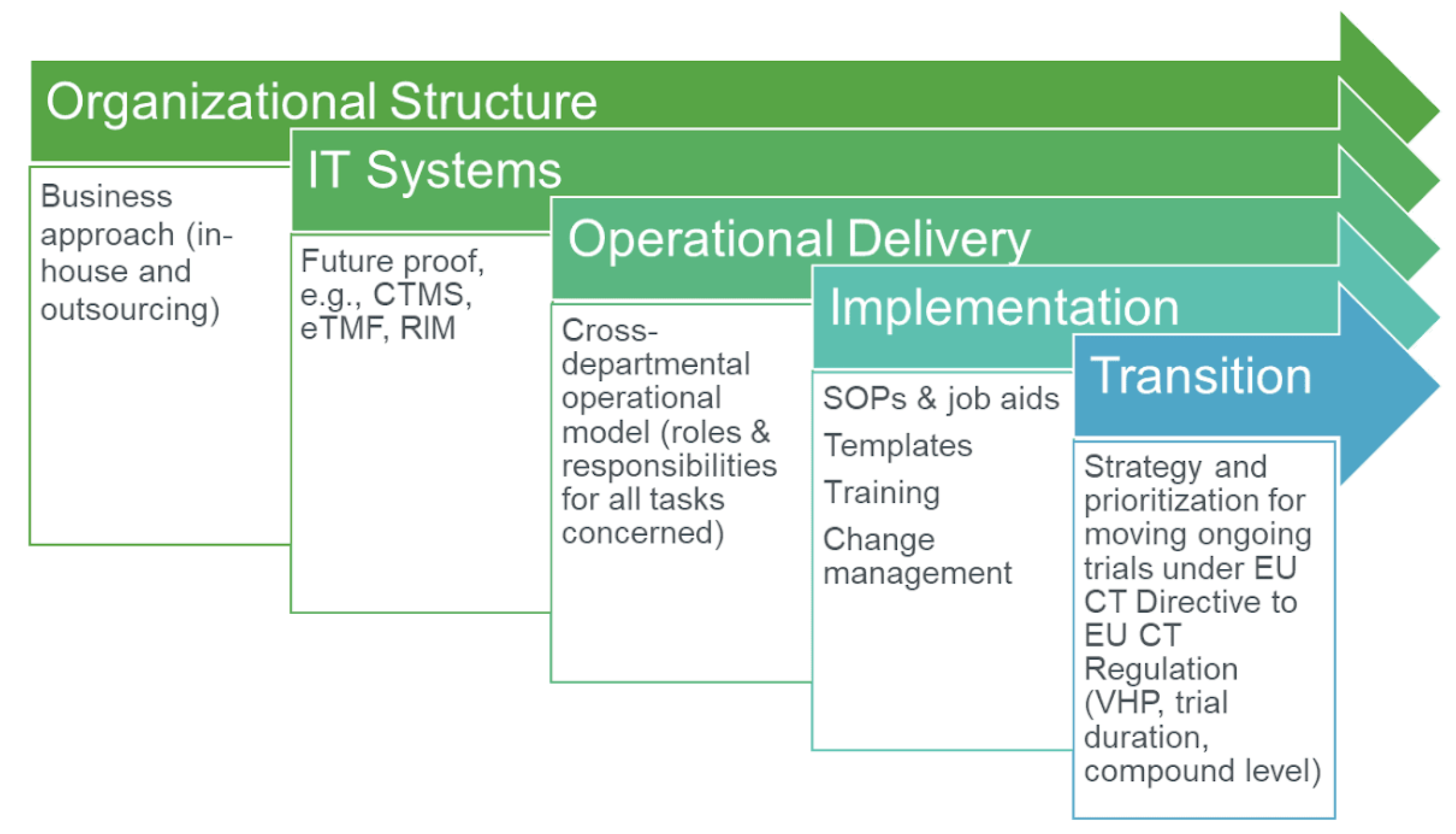
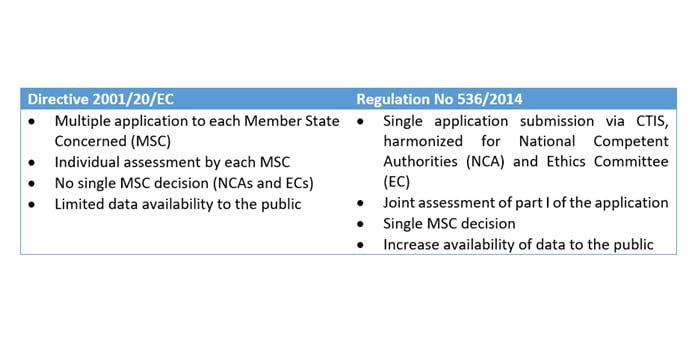
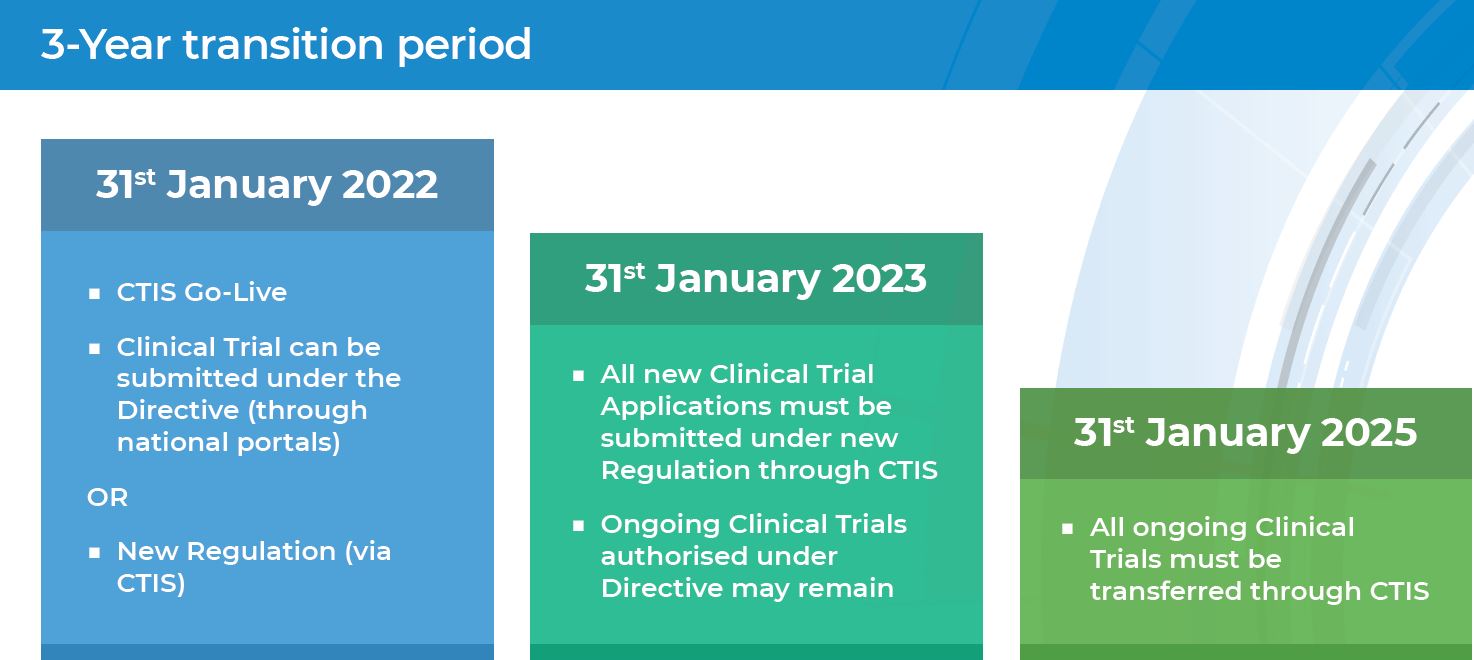
Buy The Pocket Guide to the EU Directives for Clinical Research: Clinical Trial Directive 2001/20/EC, GCP Directive 2005/28/EC, GMP Directive 2003/94/ EC Book Online at Low Prices in India | The Pocket Guide

Guide to Clinical Trials Authorised for Conduct under the Clinical Trials Directive (Council Directive 2001/20/EC) in Ireland

Exploring the Impact of the New European Directive on the Pharmaceutical Industry - Clinical Trials Arena
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials