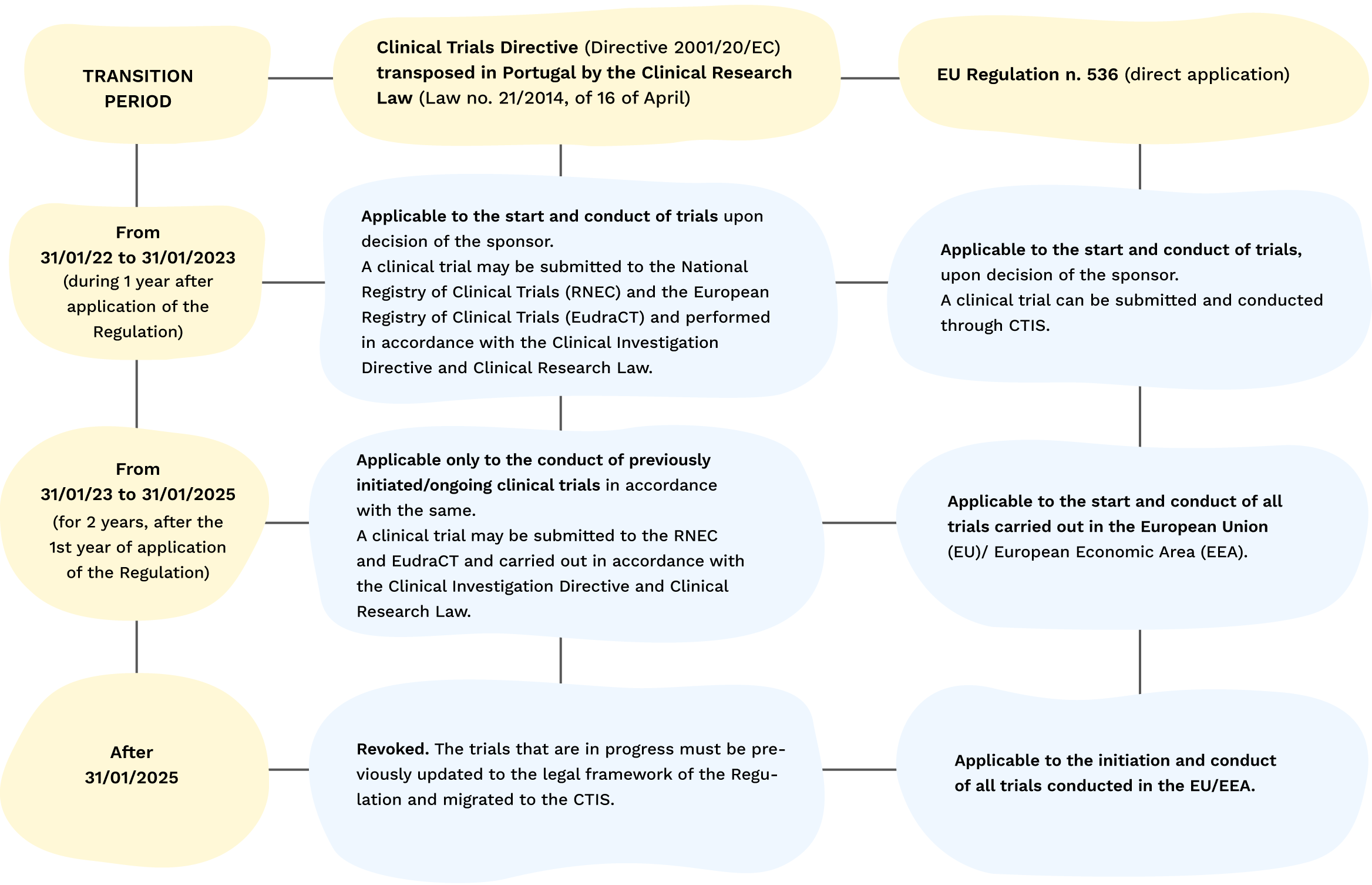
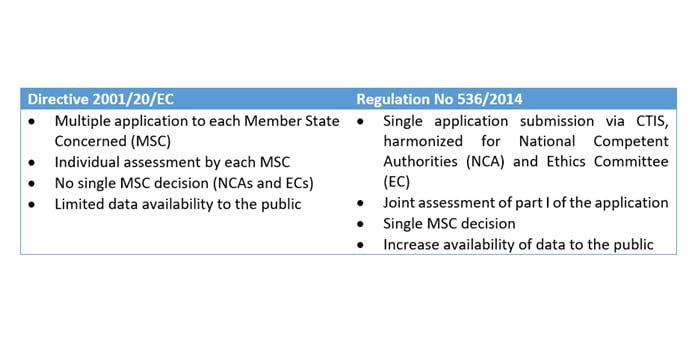
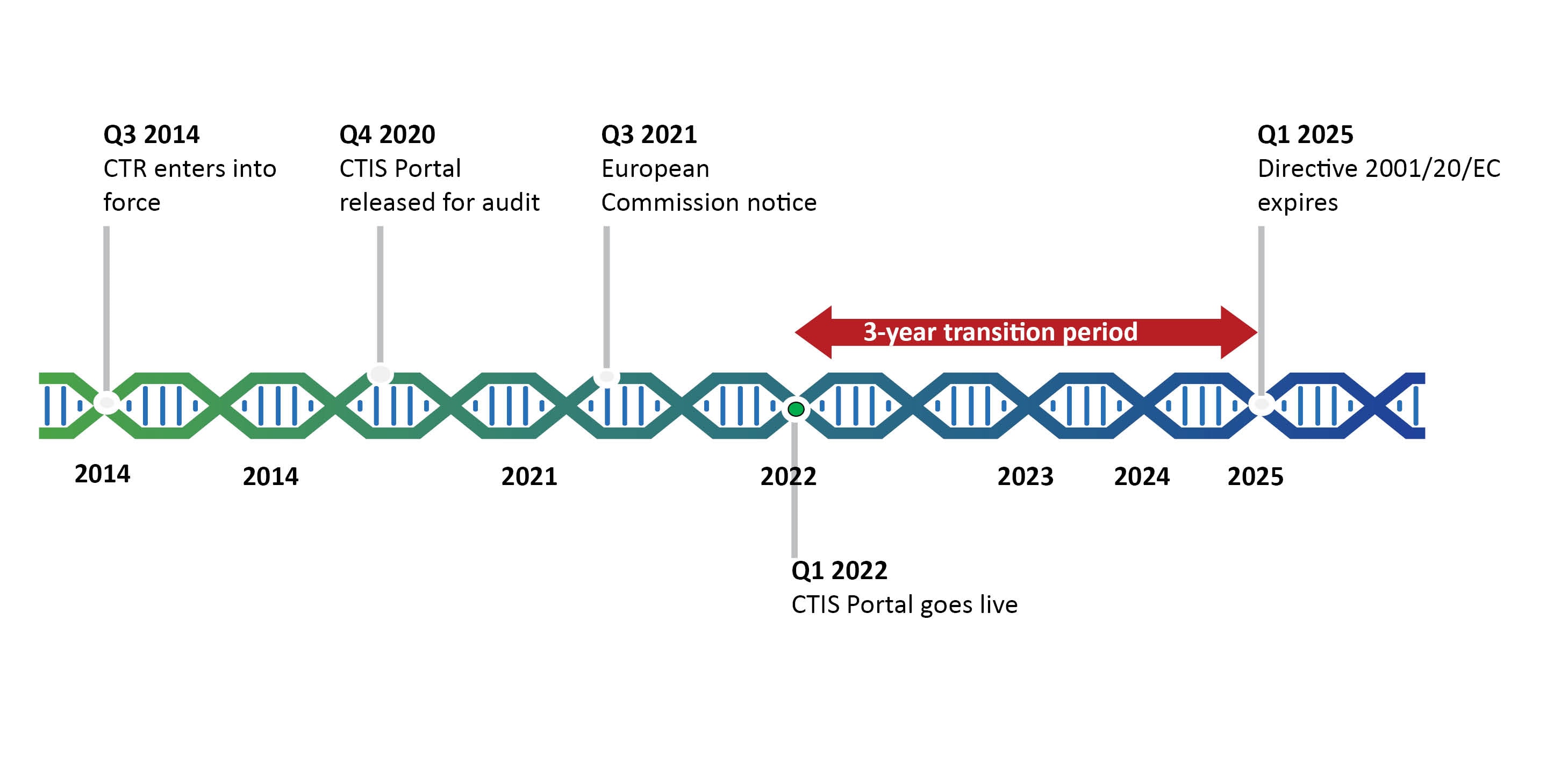
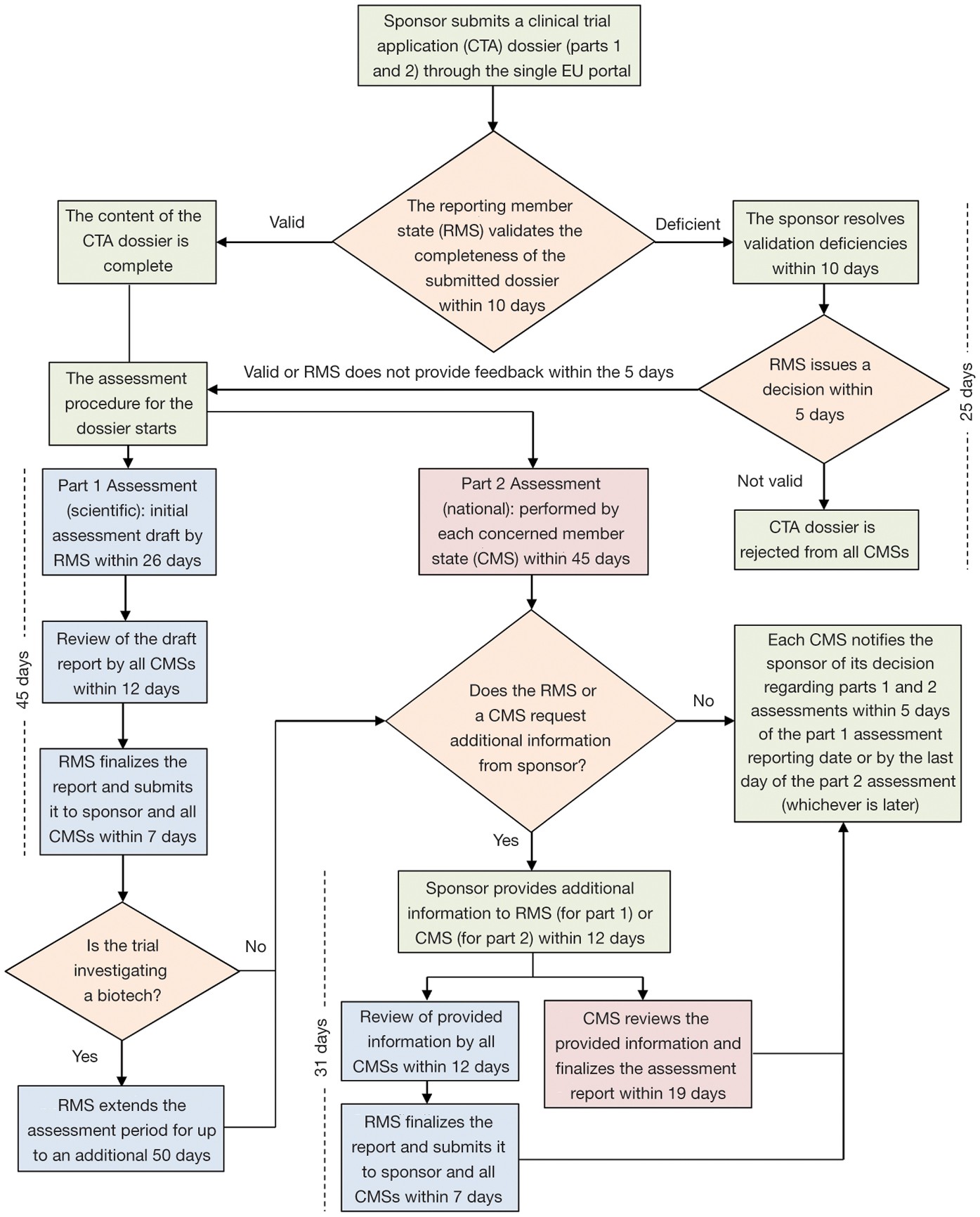
Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials

Timeline impact assessment and Revision of Directive 2001/20/EC (see... | Download Scientific Diagram

Exploring the Impact of the New European Directive on the Pharmaceutical Industry - Clinical Trials Arena

Book 6: 2023 Clinical Trials in The EU: Selected Legislation, Guidelin – Clinical Research Resources, LLC