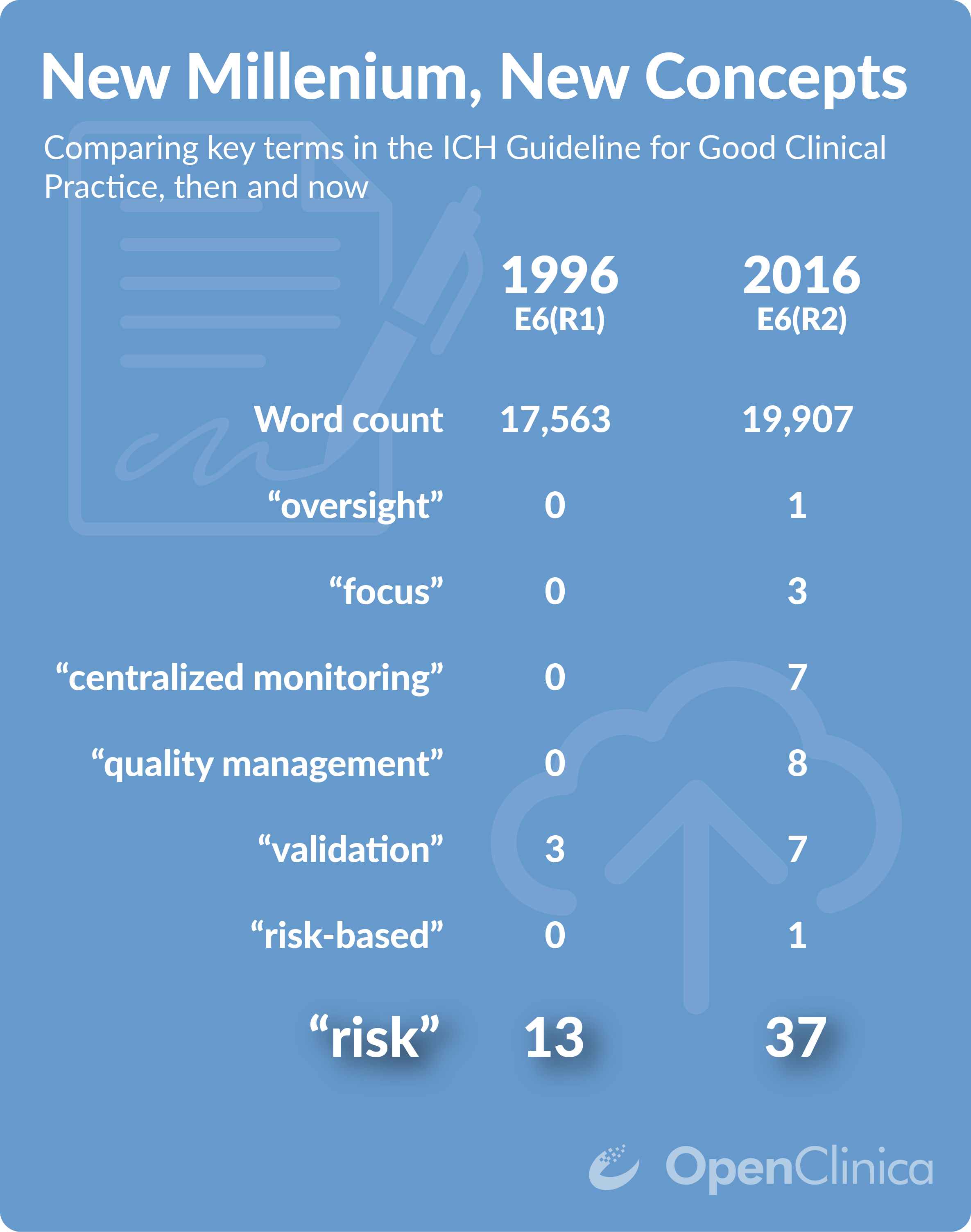
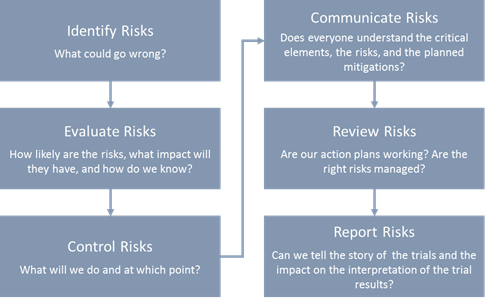
E6(R2) Good Clinical Practice Integrated Addendum to ICH E6(R1) - E6(R2) Good Clinical Practice: - Studocu

ICH GCP Guidelines E6 Revision, R2 Addendum - Changes Impacting Sponsors-CRO-Sites - Webinar Compliance

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Final ICH GCP E6 R2 Addendum: Overview of Changes Impacting Sponsors/CROs/ Clinical Investigator/Site - YouTube