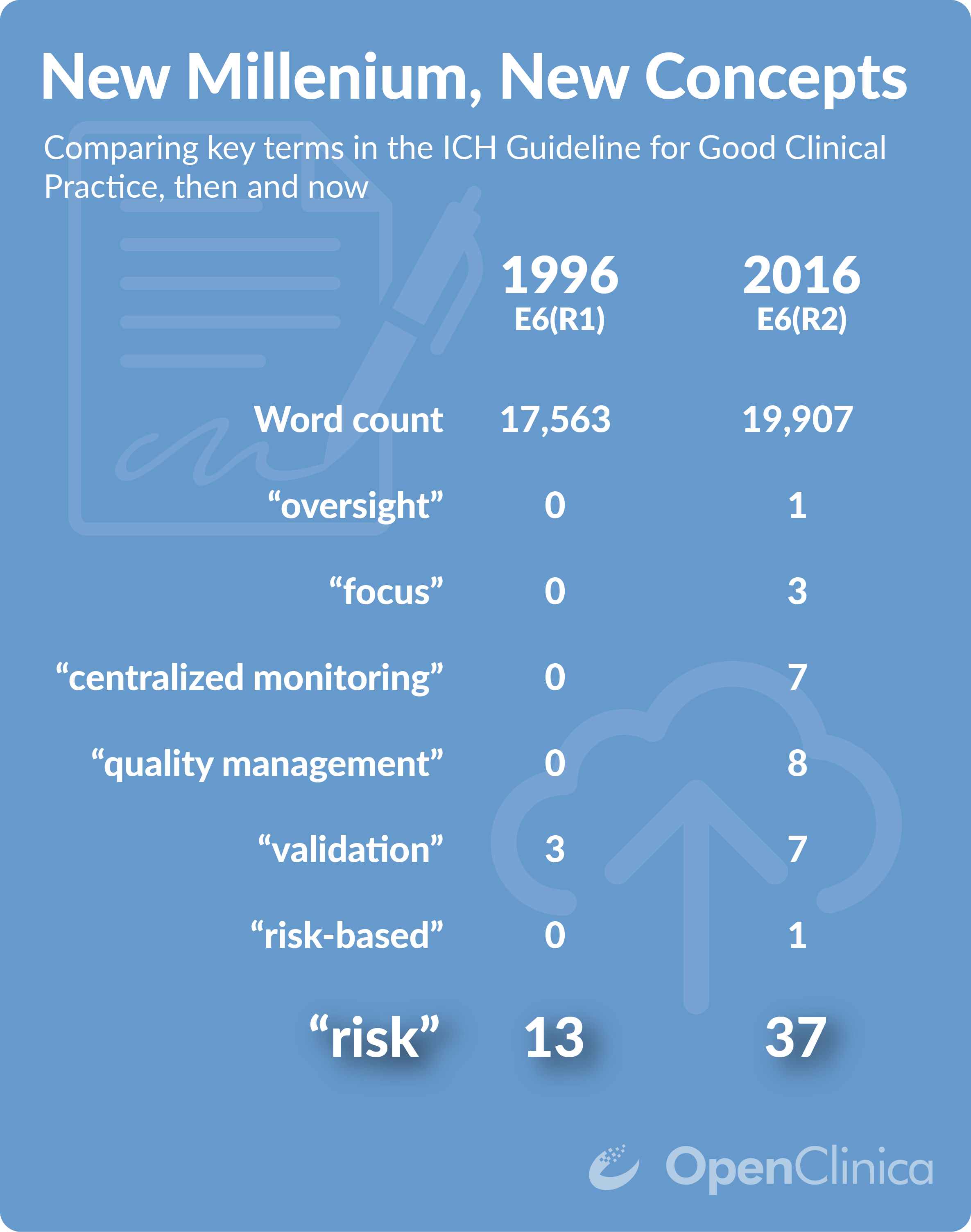
ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

E6(R2) Good Clinical Practice Integrated Addendum to ICH E6(R1) - E6(R2) Good Clinical Practice: - Studocu

Introduction To Investigators Responsibilities With Good Clinical Practice | PDF | Institutional Review Board | Clinical Trial






.jpg)